|
>>
|
No. 3139
[Edit]
File
167700063760.png
- (25.86KB
, 359x389
, 32.png
)
A brief overview
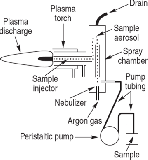
ICP-MS can analyze liquid and solid samples. An ICP-MS system consists of the following: sample introduction system, ICP torch, interface, vacuum system, lens, quadrupole mass spectrometer, detector, and data management system. First, the sample is introduced into the sample introduction system. The nebulizer, which is the first component of the sample introduction system, converts the sample into small droplets. The small droplets are then passed into the next component of the sample introduction system called the spray chamber. From here, the sample is passed through the ICP torch that contains the plasma, the ion source for ICP-MS. As the sample is ionized in the plasma, it is then passed through an interface into the mass spectrometer. The ions from the sample are then focused through a series of ion lenses and then pass into the quadrupole mass spectrometer, which separates the ions from the sample according to their mass-tocharge ratio. Following the separation, a detector measures the separated ions. Afterward, the data are analyzed by a computerized data system. Figure 2 illustrates the elements determined by ICP-MS and the approximate detection capability.3
The evolution of ICP-MS
Since its introduction in 1983, the ELAN 250 has rapidly evolved over the past 25 years. Figure 3 illustrates the evolution of the ELAN family of instruments. This first ICP-MS instrument required a considerable amount of expertise to operate. Manual adjustments were required of control knobs, lenses, etc. Instrumentation has evolved through continuous efforts up through the 1990s to the point where ICP-MS instruments are now highly automated, stable, and suitable for routine analysis. “The end of 1985 marked the watershed year,” said Dr. Andy Boorn, President of MDS Sciex. At the end of 1985, a booming 30 units were sold. “Wow, this is going to be big,” thought Dr. Boorn. Although more than 900 units are now sold every year, at that time, the level of sales in the first year marked the indication of the true need and future success of ICP-MS.
|